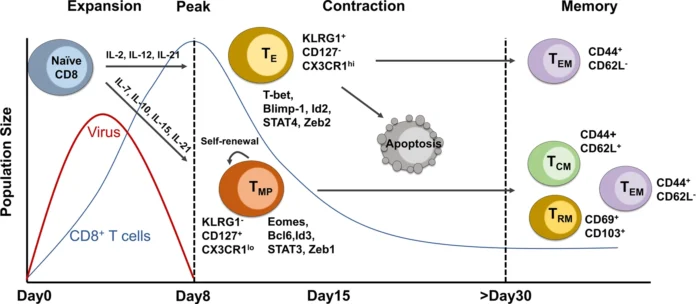
In a breakthrough that reshapes the understanding of immune system dynamics, researchers from the Technical University of Munich (TUM) and Helmholtz Munich have identified that the human body begins preparing for severe disease scenarios far earlier than previously believed. Even in cases of mild or moderate infections, the body produces a subset of T cells predisposed to exhaustion—a phenomenon previously associated only with chronic infections and tumors.
What Are T Cells and Why Are They Important?
T cells are essential components of the adaptive immune system. They help eliminate infected or malignant cells, orchestrate immune responses, and regulate the immune system’s intensity. Among them are various subtypes, each adapted for specific tasks—from cytotoxic T cells that destroy infected cells to helper T cells that guide immune coordination.
However, under prolonged or intense stimulation—such as during chronic infections or in the tumor microenvironment—some T cells enter a state known as exhaustion. In this state, they lose their effectiveness, potentially compromising immune defense. This condition also poses a major hurdle in cancer immunotherapy, where the immune response needs to remain strong and persistent.
Until now, T cell exhaustion was largely considered a late-stage phenomenon, developing only during long-lasting, high-burden infections or cancer. The new findings challenge this assumption.
New Evidence: Early T Cell Exhaustion
The study, led by Professor Dietmar Zehn, Chair of Animal Physiology and Immunology at TUM, demonstrates that T cell exhaustion begins at the earliest stages of infection—even in moderate or uncomplicated cases. According to Zehn, “We were able to show that the body prepares T cell subtypes that are predisposed to exhaustion even in early infection phases of moderate diseases.”
This suggests that the immune system prepares multiple contingencies in advance, including the possibility of dampening its own response if the infection becomes prolonged or too aggressive.
Strategic Immune Planning: Preparing for All Outcomes
According to the researchers, the immune system appears to assemble a diversified pool of T cells during the early infection window. This heterogeneous response likely includes:
READ MORE: A New Hope for Lyme Disease Prevention
- Effector T cells for immediate pathogen elimination
- Memory T cells for long-term immunity
- Exhaustion-prone T cells that can suppress overactive immune responses
Such a strategy gives the immune system flexibility. It can escalate, modulate, or terminate its defense depending on how the disease evolves. This could explain why some infections are resolved without complications, while others are self-limited to prevent immune system-induced damage.
“This layered response may be an evolutionary adaptation,” Zehn notes, “allowing the host to better balance immune control and tissue preservation.”
Implications for Immunotherapy and Disease Management
The implications of these findings are vast. As Professor Zehn explains, “Our results expand the classic idea of the development of T cell exhaustion. We therefore assume that our observations will help to further decipher the mechanisms behind T cell exhaustion.”
By deepening our understanding of when and how exhaustion-prone T cells are generated, this research could help:
- Improve cancer immunotherapies by preventing premature T cell exhaustion, making treatments more durable.
- Mitigate autoimmune disorders or cytokine storms (such as those observed in severe COVID-19), by controlling overzealous immune responses.
- Enhance vaccine design by steering immune responses away from exhaustion-prone pathways.
The insights also suggest that biomarkers of T cell exhaustion could be present far earlier in the disease than assumed. This may open doors for early diagnostic tools that assess immune readiness or vulnerability, especially in high-risk patients.
A Paradigm Shift in Immunology
This study marks a significant shift in our understanding of immune function. Rather than being a binary state—effective versus exhausted—T cell function exists on a spectrum, shaped by early signals and preemptive cellular programming.
This nuanced perspective aligns with recent theories proposing that T cell exhaustion is not solely a failure, but rather a protective mechanism to prevent autoimmunity or collateral tissue damage. It may serve as a form of immune circuit breaker, strategically employed by the body when necessary.
Future Research Directions
Building on this foundational work, the researchers at TUM and Helmholtz Munich aim to:
- Identify the molecular triggers that guide early differentiation into exhaustion-prone T cells.
- Explore whether this process can be modulated pharmacologically or genetically.
- Investigate whether early exhaustion signatures can be detected in patients with acute infections, cancers, or autoimmune conditions.
Conclusion
The discovery that T cells destined for exhaustion are present from the onset of infection marks a critical advance in immunology. It reshapes how scientists and clinicians understand immune readiness, disease progression, and therapeutic potential. This research offers new hope for fine-tuning the immune response—making it strong when necessary, and restrained when it must be.
As global efforts continue to combat cancer, viral pandemics, and immune disorders, the ability to anticipate and influence immune trajectories could redefine the future of personalized medicine and immunological resilience.