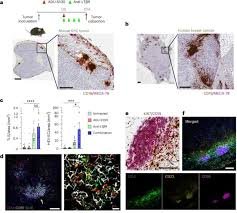
Researchers at Texas A&M University Health Science Center have made a breakthrough in understanding how an aggressive kidney cancer hijacks RNA to build molecular growth hubs—and how those hubs can be dismantled. In a rare form of kidney cancer known as Translocation renal cell carcinoma (tRCC), the team found that fusion genes (specifically TFE3 oncofusions) trigger RNA to form liquid-like condensates inside cell nuclei. These condensates act as “engine rooms” that activate tumor-promoting genes. (ScienceDaily)
What’s particularly exciting is that the scientists didn’t stop at discovery. They developed a molecular switch—a designer nanobody fused to a dissolver protein—that, when triggered, disrupted these hubs and halted tumor growth in lab dishes and in mouse models. (Vital Record) This opens up new avenues for therapies targeting condensate formation in cancers, especially those driven by fusion proteins.
How RNA Builds Tumor Hubs in tRCC
RNA is usually a messenger molecule, but in tRCC the story is very different. The fusion proteins created by TFE3 gene rearrangements recruit RNA not just to carry information, but to assemble the structural basis of these growth hubs. Within the nucleus, RNA molecules condense into droplet-like clusters—or “droplet hubs”—that gather together key proteins and gene-activating machinery. (ScienceDaily)
These hubs serve as transcriptional command centers. The RNA condensates bring together fusion proteins, RNA-binding proteins (such as PSPC1), and genes into spatial proximity, thus elevating the expression of genes that drive tumor growth. The research team used multiple advanced methods including CRISPR tagging, SLAM-seq, CUT&Tag, RIP-seq and proteomics to trace the steps of how the fusion proteins bind DNA/RNA, how RNA participates in the structure, and which proteins stabilize the hubs. (ScienceDaily)
Key steps uncovered
- Fusion gene products of TFE3 generate abnormal hybrid proteins. (Vital Record)
- These fusion proteins recruit RNA molecules to scaffold condensation into droplets. (ScienceDaily)
- RNA-binding protein PSPC1 stabilises these droplets and enhances hub formation. (Vital Record)
- The hubs bring together gene targets, enabling high activity of tumour-promoting genes. (ScienceDaily)
Because tRCC so often affects children and young adults and lacks effective therapies, this mechanistic insight is a major step forward. (EurekAlert!)
Disassembling the Engine Rooms: A New Therapeutic Strategy
The critical question: once you identify these hubs, can you shut them down? The answer from this study is yes—at least in lab and animal models. The researchers engineered a chemogenetic tool based on a nanobody that targets the fusion protein and carries a “dissolver” domain. When activated chemically, the dissolver breaks apart the RNA-based condensates and dismantles the hubs. Tumour growth was significantly reduced in cell culture and mouse models. (Vital Record)
Why this matters
- It gives a new target: condensate formation rather than just gene mutation or protein inhibition.
- It offers precision: the tool is designed to engage the fusion protein specifically, thereby limiting off-target effects.
- It creates a platform: since many pediatric and adult cancers are driven by fusion proteins, this strategy could extend beyond tRCC. (EurekAlert!)
Implementation details
Here is how the tool works in practice:
- A nanobody binds the TFE3 fusion protein inside cancer cells.
- The nanobody is fused to a dissolver protein that remains inactive until triggered.
- A chemical activator is introduced, prompting the dissolver to melt the RNA-based droplets.
- The hubs collapse, gene activation drops, cell growth slows or stops.
Because of its engineered, inducible nature this approach shows promise for controlled therapy with fewer side effects. For clinicians and researchers, the actionable insight is: explore condensate disruption as a therapeutic axis, especially in fusion-driven cancers.
Table: Summary of Key Findings and Therapeutic Implications
| Component | Role in tRCC Mechanism | Therapeutic Implication |
|---|---|---|
| TFE3 oncofusion | Creates abnormal hybrid transcription factor driving tumor growth | Primary target for nanobody/dissolver tool |
| RNA condensates (“droplet hubs”) | Structural scaffolds that bring together proteins & genes | Disruption halts hub function and gene activation |
| PSPC1 (RNA-binding protein) | Stabilises hubs and enhances oncogenic activity | Potential co-target to destabilise condensates |
| Nanobody-based chemogenetic tool | Engineered switch that dissolves hubs upon activation | Novel treatment modality offering precision and control |
| Model systems (cells & mice) | Demonstrated halt of tumour growth after hub dissolution | Preclinical validation; next step is translational research |
What This Means for Patients and the Research Community
This discovery has practical implications. For clinicians treating pediatric kidney cancers like tRCC, this research suggests a new class of intervention is possible. It highlights the importance of identifying the molecular architecture of cancers—not just the mutations but how those mutations reorganise the cell’s internal structures.
For researchers, the study provides actionable recommendations:
- Screen other cancers driven by fusion proteins for similar condensate formation mechanisms.
- Develop additional nanobody-dissolver systems tailored to different fusion proteins.
- Investigate the safety, dosage and activation mechanisms of chemogenetic dissolvers in larger animal models and eventually in humans.
- Consider combination therapies: disrupting hubs plus existing chemotherapy or immunotherapy may yield synergistic effects.
Importantly, for patients and families, this work offers hope. tRCC accounts for nearly 30 % of renal cancers in children and teens, yet effective treatments are few. (EurekAlert!) By targeting the structural basis of tumour growth, there is potential for therapy that is both more effective and less toxic than broad-acting chemotherapy.
In short, this is not just a lab finding—it’s a blueprint for a new therapeutic direction.
Trending FAQ
Q: What is translocation renal cell carcinoma (tRCC)?
A: It’s a rare form of kidney cancer that arises mainly in children and young adults, characterised by chromosomal translocations involving the TFE3 gene.
Q: How are these “droplet hubs” formed inside cancer cells?
A: The TFE3 fusion proteins recruit RNA molecules which condense into liquid-like structures, clustering transcription machinery and gene targets together to amplify tumour growth.
Q: What exactly did the chemogenetic tool do?
A: The tool uses a nanobody that binds the fusion protein and a dissolver domain activated by a chemical trigger. Once activated, it breaks up the RNA condensates and shuts down the growth hubs.
Q: Could this approach apply to other cancers?
A: Yes. Many cancers are driven by fusion proteins or abnormal condensate formation. The principle of disrupting structural hubs may be broadly relevant.
Q: What are the next steps before this becomes a therapy?
A: Further preclinical testing to assess safety, dosage, delivery, biodistribution and off-target effects. Then possibly early phase human trials.
Q: Does this replace existing therapies?
A: Not yet. It complements them. Eventually it may become part of combination regimens, but more research is required.
Q: Are there known risks to targeting condensate formation?
A: Because condensate dynamics are part of normal cell biology, precision targeting is crucial to avoid interfering with healthy cell functions. That is why inducible, selective tools are important.
This research marks a substantial leap forward in cancer biology and therapeutic development. By uncovering how RNA builds tumour-driving hubs and creating a tool to dismantle them, the Texas A&M team has opened a promising path. Investigators, clinicians and patients alike should watch this space—this might be the start of a new era in precision oncology.