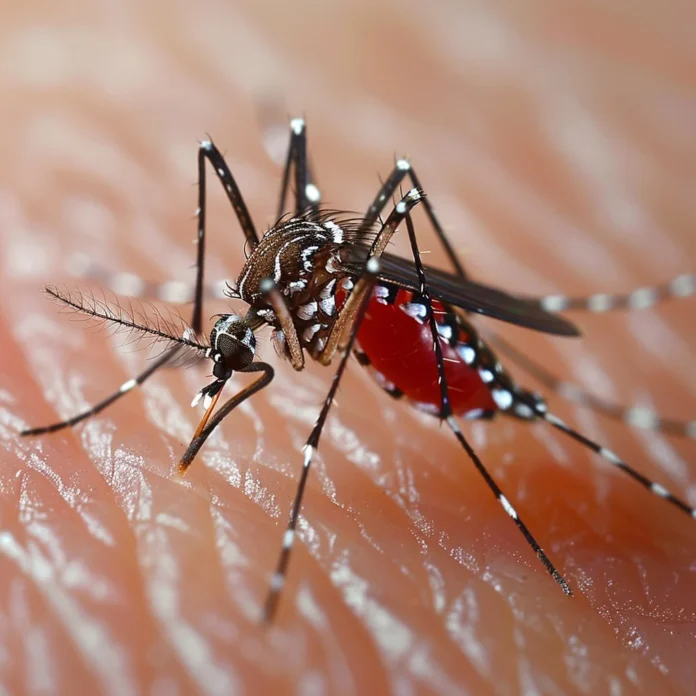
Dr Véronique Paris, a medical entomologist at the University of Melbourne, takes an unorthodox approach to studying mosquito-borne diseases: she feeds live mosquitoes on her own blood. In weekly lab sessions, she slides her bare arm into a mesh enclosure housing hundreds of mosquitoes—allowing them to bite her in order to maintain healthy colonies and collect eggs for experiments. Her hands-on method may seem extreme, but it has become an essential component of her efforts to understand and curb the spread of pathogens such as the flesh-eating Buruli ulcer.
Cultivating a Mosquito Colony by Hand
Feeding Frequency and Technique
To keep her mosquito colonies vigorous, Dr Paris feeds them on her arm approximately once a week. “For most of the ones I feed, I just feel a little sensation,” she explains. When conducting key experiments or requiring additional egg production, she increases feeding sessions to as many as four times in seven days. Each session lasts only a few minutes, during which the mosquitoes feed on capillaries close to the skin surface. Immediately afterward, she resists scratching for at least an hour to avoid exacerbating the itching.
Health and Safety Precautions
Dr Paris emphasizes that feeding on human volunteers is voluntary and not compulsory for her students or colleagues. “There will always be volunteers around that can do that, so no one has to feed mosquitoes if they don’t want to,” she says. The lab follows strict biosafety protocols to ensure researchers do not acquire infections; only non-infected mosquito strains are used in routine colony maintenance. When studying disease transmission, separate high-containment facilities and additional personal protective equipment are employed.
The Scientific Rationale for Self-Feeding
Maintaining Laboratory Strain Fidelity
Regular blood meals are critical for female mosquitoes to mature eggs. While synthetic membrane feeders can substitute in many labs, Dr Paris prefers direct feeding because it yields higher egg counts and preserves natural feeding behaviors. “If I’m running an experiment or want to collect more eggs for upcoming work, direct feeding can increase reproductive output significantly,” she notes.
Ensuring Consistency Across Experiments
Mosquito feeding behavior can affect experimental outcomes, especially when investigating disease transmission or vector competence. By personally feeding her colonies, Dr Paris controls for variation in host odor, temperature and blood composition—all factors that influence mosquito biology. This consistency helps produce reliable data on pathogen uptake, incubation periods and transmission efficiency.
Focus on Mosquito-Borne Buruli Ulcer
Rising Case Numbers in Australia
Buruli ulcer, caused by the environmental bacterium Mycobacterium ulcerans, has surged in southeastern Australia. Cases in Victoria rose from 135 in 2020 to 238 in late 2023, according to state health data. The disease causes severe skin ulcers, tissue loss and long-term disability if untreated. While possums serve as animal reservoirs, uncertainty around transmission to humans has hampered control efforts.
Linking Mosquitoes to Disease Transmission
A landmark 2024 study provided the first strong evidence that mosquitoes can transfer M. ulcerans from animal hosts to people. Building on this finding, Dr Paris aims to unravel exactly how mosquitoes acquire the bacterium and pass it on. Do pathogens multiply within the insect gut? Are bacteria deposited on the skin during probing? By maintaining healthy, well-fed colonies, she can design controlled bite-challenge experiments to answer these questions.
Modeling Infection Dynamics
In one ongoing project, Dr Paris exposes colony mosquitoes to infected possum blood in an artificial feeder, then allows them to feed on a mouse model. By tracking bacterial load over time in both insect and mammalian hosts, her team assesses how bacterial survival and migration through mosquito tissues contributes to transmission risk. These insights can guide public health interventions, from targeted mosquito control near wildlife reservoirs to novel repellents or barrier methods.
Engaging the Public via Social Media
Choosing Instagram over Academic Platforms
With more than 13,000 followers on Instagram, Dr Paris shares behind-the-scenes glimpses of her work. She intentionally left professional networks such as LinkedIn to reach audiences “not necessarily already looking for scientific content.” Videos of her volunteering for mosquito bites, laminated infographics on disease ecology and Q&A sessions demystify entomological research and foster community dialogue.
Educational Impact and Outreach
Follower questions range from basic biology (“Why do mosquitoes itch?”) to practical advice (“How can I reduce breeding sites in my backyard?”). Dr Paris often fields inquiries during live streams as she inspects larval habitats or counts eggs under a microscope. These interactions have spurred local awareness campaigns in Melbourne suburbs experiencing Buruli ulcer cases, prompting residents to eliminate standing water and report possum sightings.
Mentoring the Next Generation
Her social outreach also inspires high school and undergraduate students to consider careers in entomology and tropical medicine. Several followers have cited her videos as motivation to join summer lab internships or university entomology clubs—an unexpected but welcome boost for a field that traditionally struggles to recruit young talent.
Field Investigations and Habitat Surveys
Identifying Breeding Sites in the Wild
Laboratory work is only one part of Dr Paris’s research. She and her postdoctoral team periodically conduct field surveys in endemic areas of Victoria, North Queensland and the Northern Territory. Using dip nets and aspirators, they collect mosquito larvae from drains, garden ponds and natural wetlands. GPS mapping of breeding hotspots helps correlate disease incidence with vector abundance and environmental factors such as rainfall and temperature.
Assessing Mosquito Species Diversity
Australia harbors around 300 mosquito species, each with different feeding preferences and vectorial capacities. During field trips, Dr Paris sets up light traps and human-landing collections (under strict ethical oversight) to sample adult populations. She then identifies specimens to species level using morphological keys and molecular barcoding, enabling her lab to maintain multiple colony lines for comparative studies.
Integrating Ecology and Epidemiology
By combining laboratory transmission experiments with field abundance data, Dr Paris constructs risk models predicting human exposure under various scenarios. For instance, prolonged wet seasons may boost certain vector species, elevating outbreak probabilities. Public health authorities can then time control measures—such as targeted larviciding or awareness campaigns—to match periods of high risk.
Towards Novel Control Strategies
Exploring Endosymbiotic Interventions
Inspired by successful use of Wolbachia bacteria to curb dengue in Aedes mosquitoes, Dr Paris is investigating whether similar symbionts could reduce M. ulcerans acquisition or survival within organisms like Culex or Anopheles. Early lab trials assess whether co-infecting mosquitoes with candidate bacteria impedes pathogen colonization without harming insect fitness.
Developing Improved Repellents and Traps
Based on her observations of species-specific feeding behaviors, Dr Paris’s team is testing new natural‐compound repellents that target chemoreceptors unique to regional vectors. They are also designing oviposition traps—baited with attractants that lure gravid females to lay eggs on treated surfaces—thereby reducing larval recruitment in endemic zones.
The Future of Mosquito Research in Australia
Expanding Surveillance Networks
Dr Paris advocates for a nationwide mosquito-borne disease surveillance network, integrating entomological, veterinary and clinical data. Such a system would enable real-time monitoring of emerging threats—from exotic dengue vectors like Aedes albopictus to zoonotic agents in wildlife. Early detection could trigger rapid vector control responses and clinical alerts, minimizing public health impacts.
Strengthening Global Collaborations
Australia’s scientific community benefits from partnerships with researchers in Southeast Asia, Africa and South America—regions where mosquito-borne pathogens like Buruli ulcer, Ross River virus and malaria are endemic. Dr Paris participates in international consortia that share methodologies, data and best practices, ensuring that Australia’s unique insights contribute to global disease control efforts.
Conclusion
Dr Véronique Paris’s willingness to serve as her own mosquito host exemplifies the dedication and ingenuity driving modern vector biology. By combining rigorous laboratory protocols with immersive fieldwork and innovative public outreach, she illuminates the complex ecology of mosquito-borne diseases in Australia. Her work not only deepens scientific understanding but also empowers communities to protect themselves—folding the very sting of a mosquito bite into a broader campaign against some of the country’s most insidious pathogens.
READ MORE: Measles Alert Issued in Sydney After Traveller from Southeast Asia Tests Positive