Owlstone Medical, a global leader in breath-based diagnostics through its proprietary Breath Biopsy® platform, has announced an equity investment of up to $2.3 million USD (approximately £1.7 million) from the Cystic Fibrosis Foundation. The funding will be used to support the development of a non-invasive breath test for the detection of Pseudomonas aeruginosa (PA) in patients with cystic fibrosis (CF).
Purpose of the Investment
The primary goal of the investment is to create a breath-based diagnostic with comparable accuracy to sputum culture, the current gold standard. The envisioned test would support both:
- Early detection of new PA infections
- Monitoring of chronic PA infections in CF patients
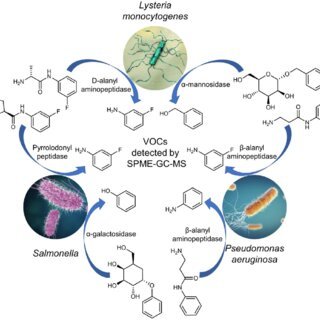
The test will rely on volatile organic compound (VOC) analysis—a core technology developed and refined by Owlstone Medical—to identify distinct chemical signatures associated with PA infection.
Focus on Chronic Infections
The initial phase of the project will concentrate on establishing scientific proof-of-principle for detecting chronic PA infection. The development aims to identify specific VOCs in breath that correlate with the presence of PA in the lungs.
READ MORE: Older Adults Thrive on Immunotherapy Despite Age Gaps
If successful, subsequent studies will explore the test’s ability to detect new infections, a critical aspect of preventative care and treatment optimization in CF patients.
Importance of Early Detection in CF
CF is a progressive genetic condition affecting over 105,000 individuals worldwide, characterized by excessive mucus accumulation in the lungs. This environment fosters bacterial infections, particularly PA, which is one of the most dangerous pathogens for people with CF.
PA is notoriously difficult to eradicate once it becomes established in the lungs. Its presence is associated with accelerated lung decline, heightened risk of hospitalization, and worse overall prognosis.
Approximately 25% of CF patients are infected with PA, and early, aggressive treatment can prevent chronic colonization and associated complications.
Limitations of Current Testing Methods
Currently, PA is diagnosed through sputum cultures, which involve analyzing mucus samples for bacterial growth. However, this method presents practical challenges, particularly for:
- Children, who often struggle to produce sputum
- Adults, who may not generate sufficient sputum due to improved airway clearance from existing CF therapies
This testing gap creates an urgent need for non-invasive, reliable alternatives, such as breath testing.
Owlstone’s Track Record in Breath-Based Diagnostics
Owlstone Medical is uniquely positioned to develop this breakthrough diagnostic tool, having demonstrated success in breath-based detection of infectious diseases through collaborations with:
- The U.S. Department of Defense
- The Gates Foundation
- Other global health partners
The company’s Breath Biopsy® platform has enabled the analysis of breath biomarkers to support early detection and precision medicine applications across a range of disease areas.
Statement from Owlstone CEO Billy Boyle
“Owlstone’s experience in the breath-based detection of infectious disease through projects with the U.S. Department of Defense, the Gates Foundation, and other partners, underpinned by our proprietary Breath Biopsy platform, places us in an excellent position to help improve outcomes for CF patients infected with Pseudomonas aeruginosa,” said Billy Boyle, Co-founder and CEO of Owlstone Medical.
He added that the data collected during this project will be integrated into the company’s growing Breath VOC Atlas — a centralized resource used to support biomarker discovery and diagnostic tool development for various diseases.
Broader Implications and Future Potential
This initiative marks a significant milestone in the use of breath testing to transform chronic disease management. If successful, the test could be extended beyond PA to identify and monitor:
- Other common respiratory pathogens, such as Staphylococcus aureus
- Broader lung microbiome signatures
- Early signs of infection flare-ups in CF and potentially non-CF bronchiectasis patients
It also aligns with global efforts to shift healthcare from reactive to proactive, using non-invasive biomarker monitoring to detect disease earlier and guide personalized treatment pathways.
Conclusion
The Cystic Fibrosis Foundation’s investment in Owlstone Medical represents a transformational opportunity to improve early diagnosis and long-term management of PA infections in CF patients. By advancing a non-invasive breath-based alternative to sputum culture, this collaboration holds promise for reducing diagnostic delays, improving patient outcomes, and lowering healthcare burdens associated with chronic lung infections.